5 Kelvin Boiling Points
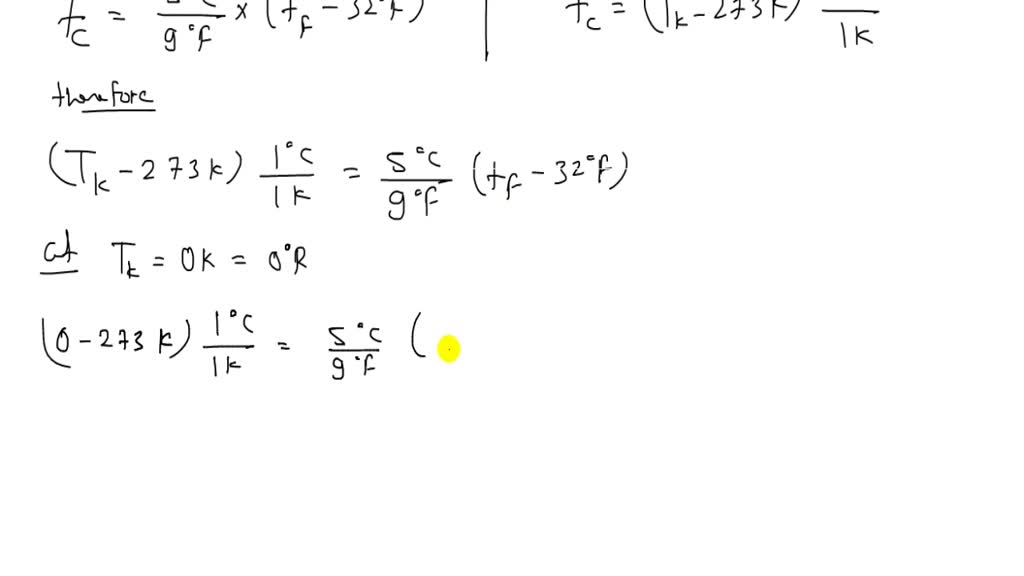
At 5 Kelvin, we’re exploring a realm of extreme cold, far below the freezing point of water and even the boiling points of most substances. To understand what happens at such low temperatures, let’s delve into the behavior of matter and how it responds to these conditions.
Introduction to Cryogenic Temperatures
Cryogenic temperatures are those below 123 Kelvin (-150°C or -238°F), and they have unique properties that distinguish them from higher temperatures. At 5 Kelvin, we’re dealing with a temperature that’s just a fraction of a degree above absolute zero, the theoretical minimum temperature possible. This is a domain where quantum mechanics starts to dominate the behavior of materials, and classical physics no longer applies.
The Importance of Boiling Points at Low Temperatures
The boiling point of a substance is the temperature at which its vapor pressure equals the surrounding pressure, causing the liquid to boil. However, at cryogenic temperatures like 5 Kelvin, the concept of boiling points becomes less straightforward. Most substances are solid at these temperatures, and their behavior is more akin to that of a quantum system than a classical liquid or gas.
Substances with Boiling Points Relevant to 5 Kelvin
While there aren’t many substances that boil at exactly 5 Kelvin, some have boiling points in the vicinity of this temperature under specific conditions. Let’s examine a few:
Helium-4: This isotope of helium has a boiling point of about 4.2 Kelvin at atmospheric pressure. It’s one of the few substances that remains liquid at such low temperatures and is crucial for cooling superconducting materials and achieving extremely low temperatures in research settings.
Hydrogen: Hydrogen gas has a boiling point of approximately 20.3 Kelvin at standard pressure, but under certain conditions, its boiling point can be adjusted. However, it’s not directly relevant to 5 Kelvin but is an example of how boiling points can vary with pressure and conditions.
Nitrogen: With a boiling point of about 77.4 Kelvin, nitrogen is another substance that, like hydrogen, doesn’t boil at 5 Kelvin but is significant in cryogenic applications. It’s often used as a coolant due to its relatively low boiling point compared to other elements.
Oxygen: Boiling at approximately 90.2 Kelvin, oxygen, like nitrogen, is crucial in cryogenics, especially in the production of liquid oxygen for industrial and medical purposes. Again, its boiling point is far above 5 Kelvin, but it’s an important element in low-temperature applications.
Neon: With a boiling point of about 27.1 Kelvin, neon is closer to the 5 Kelvin mark but still significantly higher. It’s used in neon signs and plasma TVs, and its low boiling point makes it useful in certain cryogenic applications.
Practical Applications of Cryogenic Temperatures
While the specific boiling points of substances at 5 Kelvin might not have direct, widespread applications, the understanding and manipulation of materials at these temperatures are crucial for various fields:
- Superconductivity: Materials that can conduct electricity with zero resistance often require cooling to near-absolute zero temperatures.
- Quantum Computing: The operation of quantum computers depends on the principles of quantum mechanics, which are more pronounced at very low temperatures.
- Cryogenic Storage: For applications like preserving biological samples or supercooling materials for specific properties, achieving temperatures near 5 Kelvin is essential.
Conclusion
Exploring the boiling points of substances at temperatures as low as 5 Kelvin offers insights into the quantum world and the unique properties of matter under extreme conditions. While few practical applications directly involve boiling at this specific temperature, the research and understanding derived from such studies are pivotal for advancing technologies that rely on cryogenic temperatures.
What is the significance of 5 Kelvin in scientific research?
+5 Kelvin is significant because it's near the absolute zero temperature, where quantum effects become more pronounced. This temperature range is crucial for studies on superconductivity, quantum computing, and understanding the behavior of matter at the quantum level.
Which substances remain liquid at temperatures around 5 Kelvin?
+One of the few substances that remains liquid at temperatures around 5 Kelvin is helium-4. It has a boiling point of about 4.2 Kelvin at atmospheric pressure and is used in cooling superconducting materials.
What are the practical applications of achieving temperatures near 5 Kelvin?
+Achieving temperatures near 5 Kelvin has practical applications in superconductivity, quantum computing, and cryogenic storage. These low temperatures are necessary for the operation of quantum computers, superconducting materials, and the preservation of biological samples.
In the realm of extreme cold, the behavior of substances is dictated by the principles of quantum mechanics, offering a fascinating area of study with significant implications for advanced technologies. Understanding the properties of matter at such low temperatures as 5 Kelvin opens doors to innovations that could transform industries and our understanding of the fundamental laws of physics.



