Trigonal Pyramidal Geometry: Understand Molecular Shapes
The realm of molecular geometry is a fascinating field that has captivated the imagination of chemists and scientists for centuries. One of the most intriguing and fundamental concepts in this field is the trigonal pyramidal geometry, a molecular shape that has far-reaching implications in understanding chemical properties and reactivity. In this comprehensive guide, we will delve into the world of trigonal pyramidal geometry, exploring its definition, characteristics, and significance in the context of molecular science.
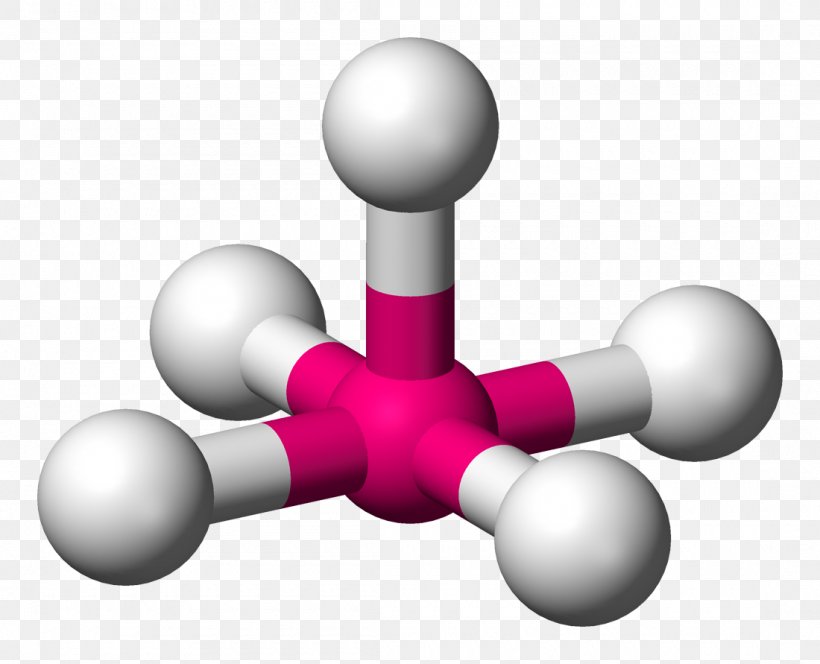
To begin with, let’s define what trigonal pyramidal geometry is. In chemistry, the term “trigonal pyramidal” refers to a molecular shape that resembles a pyramid with a triangular base. This geometry is typically observed in molecules with a central atom bonded to three other atoms, with one lone pair of electrons occupying the fourth position. The resulting shape is a pyramid with a triangular base, where the central atom is situated at the apex, and the three bonded atoms are located at the corners of the base.
The trigonal pyramidal geometry is a fundamental concept in molecular science, as it provides valuable insights into the chemical properties and reactivity of molecules. By understanding the spatial arrangement of atoms in a molecule, scientists can predict its behavior, stability, and potential interactions with other molecules.
One of the key characteristics of the trigonal pyramidal geometry is its asymmetry. Unlike the symmetrical tetrahedral or octahedral geometries, the trigonal pyramidal shape is inherently asymmetrical, with the lone pair of electrons occupying a distinct position. This asymmetry has significant implications for the molecular properties, such as dipole moment, polarity, and chemical reactivity.
| Molecular Geometry | Characteristics | Examples |
|---|---|---|
| Tetrahedral | Symmetrical, 4 bonded atoms | Methane (CH4), Ammonia (NH3) |
| Trigonal Pyramidal | Asymmetrical, 3 bonded atoms, 1 lone pair | Phosphine (PH3), Arsenic Trichloride (AsCl3) |
| Octahedral | Symmetrical, 6 bonded atoms | Sulfur Hexafluoride (SF6), Molybdenum Hexacarbonyl (Mo(CO)6) |

The trigonal pyramidal geometry is commonly observed in molecules with a central atom from the p-block of the periodic table, such as phosphorus, arsenic, and antimony. These elements tend to form molecules with a lone pair of electrons, which occupies a distinct position in the trigonal pyramidal geometry. Examples of molecules with this geometry include phosphine (PH3), arsenic trichloride (AsCl3), and stibine (SbH3).
Identifying Trigonal Pyramidal Geometry
- Determine the central atom and its valence electrons
- Count the number of bonded atoms and lone pairs
- Apply VSEPR theory to predict the molecular shape
- Verify the asymmetry of the molecular shape
In conclusion, the trigonal pyramidal geometry is a fundamental concept in molecular science, providing valuable insights into the chemical properties and reactivity of molecules. By understanding the characteristics and significance of this geometry, scientists can better predict the behavior of molecules and design new materials with specific properties.
What is the main characteristic of the trigonal pyramidal geometry?
+The main characteristic of the trigonal pyramidal geometry is its asymmetry, which arises from the presence of a lone pair of electrons occupying a distinct position.
Which elements tend to form molecules with a trigonal pyramidal geometry?
+Elements from the p-block of the periodic table, such as phosphorus, arsenic, and antimony, tend to form molecules with a trigonal pyramidal geometry.
What is the significance of the trigonal pyramidal geometry in molecular science?
+The trigonal pyramidal geometry provides valuable insights into the chemical properties and reactivity of molecules, allowing scientists to predict their behavior and design new materials with specific properties.
By exploring the world of trigonal pyramidal geometry, we can gain a deeper understanding of the intricate relationships between molecular structure, chemical properties, and reactivity. As we continue to advance our knowledge of molecular science, the significance of this geometry will only continue to grow, enabling us to develop new materials, design more efficient processes, and unlock the secrets of the molecular world.

