Calculating Molar Absorptivity Easily

The concept of molar absorptivity is a fundamental principle in chemistry, particularly in the field of spectroscopy. It is a measure of how strongly a chemical species absorbs light at a specific wavelength. Calculating molar absorptivity is crucial for understanding the interaction between matter and electromagnetic radiation, which has numerous applications in fields such as chemistry, biology, and physics.
To begin with, let’s define molar absorptivity. It is represented by the symbol ε (epsilon) and is defined as the absorbance of a solution of a substance, divided by the product of the molar concentration of the substance and the path length of the light through the sample. The formula to calculate molar absorptivity is:
ε = A / (c * l)
where: - ε is the molar absorptivity (usually expressed in units of L mol^-1 cm^-1 or M^-1 cm^-1), - A is the absorbance of the solution (a dimensionless quantity), - c is the molar concentration of the substance (expressed in units of moles per liter, M), - l is the path length of the light through the sample (expressed in units of centimeters, cm).
Understanding the Components
Absorbance (A): This is a measure of the amount of light absorbed by the sample. It can be measured using a spectrophotometer, which is an instrument designed to measure the interaction between light and the material being tested. The absorbance is related to the concentration of the absorbing species and the path length of the light through the sample via the Beer-Lambert law.
Molar Concentration ©: The molar concentration of a solution is the number of moles of solute per liter of solution. It is a critical factor in determining the absorbance of a solution because the amount of light absorbed is directly proportional to the concentration of the absorbing species.
Path Length (l): The path length refers to the distance that the light travels through the sample. In most spectrophotometric measurements, the path length is fixed and known, typically 1 cm for standard cuvettes used in UV-Vis spectroscopy.
Practical Calculation of Molar Absorptivity
To calculate the molar absorptivity of a substance, one would typically follow these steps:
Prepare Solutions: Prepare several solutions of the substance at different, known concentrations. Ensure that these solutions are in a solvent that does not absorb light significantly at the wavelength of interest.
Measure Absorbance: Use a spectrophotometer to measure the absorbance of each solution at the specific wavelength where the substance is known to absorb light. It’s crucial to use the same path length (typically 1 cm) for all measurements.
Plot Absorbance vs. Concentration: Plot the absorbance values against the respective concentrations. According to the Beer-Lambert law, this plot should be linear, with the slope of the line representing the product of the molar absorptivity (ε) and the path length (l).
Calculate Molar Absorptivity: From the linear plot, determine the slope. Knowing the path length (usually 1 cm for standard cuvettes), you can calculate the molar absorptivity using the formula ε = slope / l.
Example Calculation
Let’s consider a hypothetical example where the absorbance of a substance at different concentrations is measured, and the following data are obtained:
| Concentration (M) | Absorbance |
|---|---|
| 0.1 | 0.25 |
| 0.2 | 0.50 |
| 0.3 | 0.75 |
| 0.4 | 1.00 |
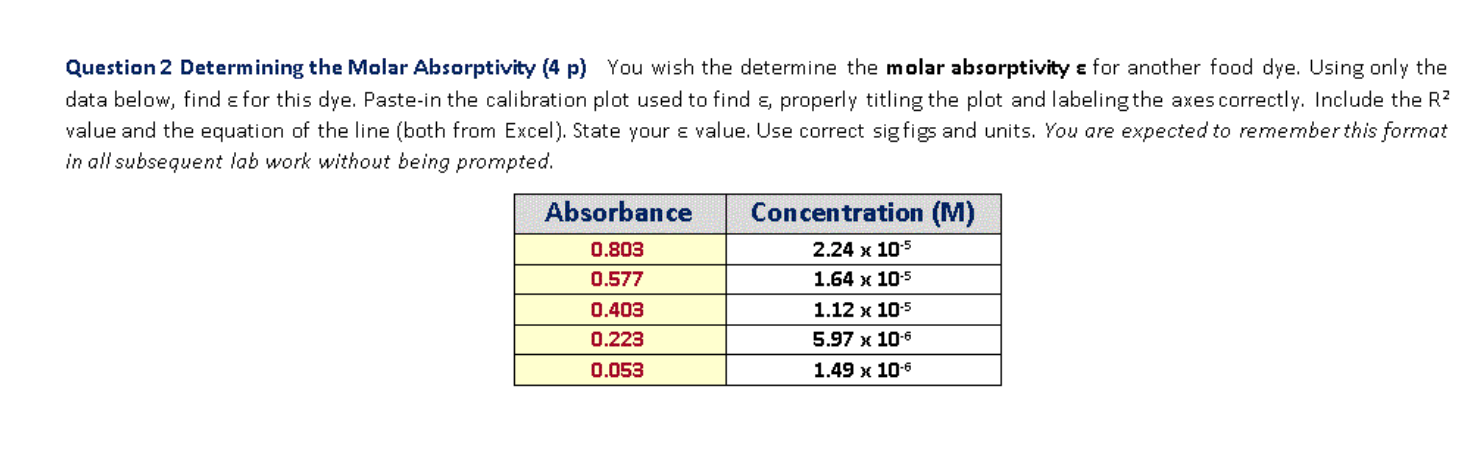
Plotting these data points, we find that the relationship is linear with a slope of 2.5 M^-1 cm^-1. Given that the path length is 1 cm, we can calculate the molar absorptivity as follows:
ε = slope / l = 2.5 M^-1 cm^-1 / 1 cm = 2.5 M^-1 cm^-1
Thus, the molar absorptivity of the substance at the wavelength of interest is 2.5 M^-1 cm^-1.
Conclusion
Calculating molar absorptivity is a straightforward process that involves measuring the absorbance of a substance at different concentrations and applying the Beer-Lambert law. It is a fundamental concept in spectroscopy and has numerous applications in analytical chemistry, allowing for the quantification of substances in solution. By understanding how to calculate molar absorptivity, researchers and scientists can better interpret spectroscopic data and apply this knowledge in various fields of study.
In practical terms, knowing the molar absorptivity of a substance can help in determining the concentration of that substance in a solution, which is essential in many chemical, biological, and environmental analyses. Moreover, molar absorptivity values are critical in the development of new spectroscopic methods for analytical purposes, underscoring its significance in the advancement of scientific research and technology.
What is the significance of molar absorptivity in chemistry?
+Molar absorptivity is significant in chemistry because it allows for the quantification of substances in solution through spectroscopic methods. It is a measure of how strongly a chemical species absorbs light at a specific wavelength, which is crucial for understanding the interaction between matter and electromagnetic radiation.
How is molar absorptivity calculated?
+Molar absorptivity is calculated using the formula ε = A / (c * l), where ε is the molar absorptivity, A is the absorbance, c is the molar concentration of the substance, and l is the path length of the light through the sample. In practice, this involves measuring the absorbance of solutions at different concentrations, plotting these values to obtain a linear relationship, and then calculating the molar absorptivity from the slope of this line.
What are the applications of molar absorptivity in science?
+The applications of molar absorptivity are diverse and include analytical chemistry, where it is used for the quantification of substances in solution. It is also crucial in the development of new spectroscopic methods, in understanding chemical reactions, and in the study of the properties of materials. Moreover, knowledge of molar absorptivity is essential in fields such as environmental science, biology, and physics, where the interaction between light and matter is of significant interest.


