12+ Essential Potassium Dot Diagram Tips
Understanding potassium’s dot diagram is crucial for grasping its chemical properties and behavior. The dot diagram, also known as the electron dot diagram, is a simple way to represent the valence electrons of an atom. For potassium, with its atomic number of 19, the dot diagram helps in understanding how it interacts with other elements to form compounds. Here are essential tips for creating and interpreting potassium’s dot diagram:
1. Start with the Atomic Number
Potassium’s atomic number is 19. This means it has 19 protons and, in its neutral state, 19 electrons. Understanding the atomic number is the first step in creating an accurate dot diagram.
2. Determine the Electron Configuration
Before drawing the dot diagram, it’s helpful to know the electron configuration of potassium. The electron configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. This tells us how the electrons are arranged in the atom.
3. Focus on Valence Electrons
The dot diagram primarily focuses on the valence electrons, which are the electrons in the outermost shell of the atom. For potassium, the valence electron is in the 4s orbital.
4. Representing the Dot Diagram
- Core: The inner electrons (1s² 2s² 2p⁶ 3s² 3p⁶) are often represented as a single entity or not shown at all to simplify the diagram.
- Valence Electron: The single electron in the 4s orbital is represented by a dot. Since potassium has one valence electron, its dot diagram is quite simple, with just one dot.
5. Understanding Reactivity
The single valence electron in potassium’s outer shell makes it highly reactive. Potassium readily loses this electron to form a positive ion (K⁺) with a +1 charge. This reactivity is a key aspect of interpreting the dot diagram.
6. Ionic Bonding
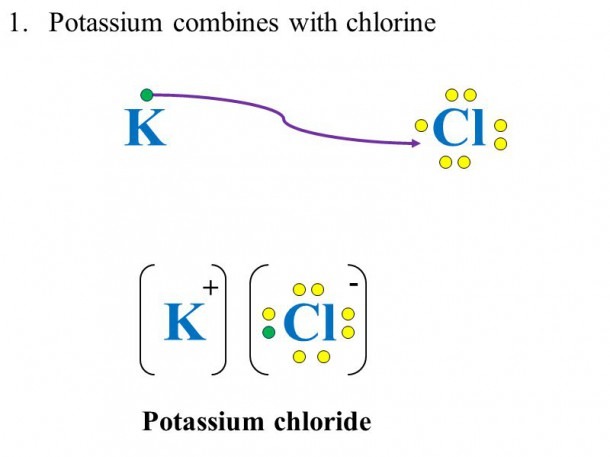
Potassium’s tendency to lose its valence electron makes it a strong participant in ionic bonding. When potassium reacts with a nonmetal like chlorine (Cl), it loses its electron to chlorine, which gains the electron to form a chloride ion (Cl⁻), resulting in the formation of potassium chloride (KCl).
7. Chemical Properties
The dot diagram helps in understanding potassium’s chemical properties, such as its high reactivity with water, releasing hydrogen gas and forming potassium hydroxide. This reactivity is due to its tendency to lose its single valence electron easily.
8. Comparison with Other Elements
Comparing potassium’s dot diagram with other elements, especially within the same group (Group 1, the alkali metals), shows similarities in reactivity due to the single valence electron in each.
9. Dot Diagram for Compounds
When potassium forms compounds, its dot diagram changes. For example, in KCl, potassium loses its electron, and chlorine gains it, so the dot diagram for potassium in this compound would show no valence electrons, while chlorine’s would show eight valence electrons, fulfilling the octet rule.
10. Limitations of the Dot Diagram
While the dot diagram is a useful tool, it has limitations. It doesn’t show the actual shape of the orbitals or the probability distribution of electrons. It’s a simplified model that is particularly useful for understanding basic chemical bonding and reactivity.
11. Visualizing Electron Transfer
The dot diagram can help visualize how electrons are transferred between atoms. In the case of potassium, visualizing the loss of its single electron to form a positive ion helps in understanding its reactivity and the formation of ionic bonds.
12. Practical Applications
Understanding potassium’s dot diagram has practical applications in fields like chemistry and materials science. For example, knowing how potassium reacts with other elements can inform the development of new materials or the improvement of existing chemical processes.
Additional Tips for Mastery
- Practice Drawing Dot Diagrams: For various elements, including those in different groups and periods of the periodic table.
- Relate Dot Diagrams to the Periodic Table: Understanding how the position of an element in the periodic table relates to its electron configuration and dot diagram.
- Apply to Chemical Reactions: Use dot diagrams to predict the products of chemical reactions and to understand the mechanism of reactions.
In conclusion, mastering the dot diagram for potassium and other elements is a foundational skill in chemistry that helps in understanding chemical properties, reactivity, and the formation of compounds. By following these tips and practicing the creation and interpretation of dot diagrams, one can gain a deeper understanding of chemistry and its applications.
What does the dot diagram of potassium represent?
+The dot diagram of potassium represents its valence electrons. Potassium has one valence electron, which is represented by a single dot in its dot diagram.
Why is potassium highly reactive?
+Potassium is highly reactive because it has a single valence electron in its outer shell, which it can easily lose to form a positive ion. This makes it highly reactive with elements that can accept an electron, such as nonmetals.
What type of bond does potassium form with nonmetals?
+Potassium forms ionic bonds with nonmetals. When potassium reacts with a nonmetal, it loses its valence electron to the nonmetal, resulting in the formation of ions that are attracted to each other, forming an ionic compound.
By understanding and applying the concepts related to potassium’s dot diagram, individuals can enhance their comprehension of chemical principles and improve their ability to predict and explain chemical phenomena.

